by Microbix | Apr 15, 2024 | Presentation
Reading Time: < 1 minute Microbix Biosystems Inc. – Our Company Today. Download The Presentation Here...
by Microbix | Apr 11, 2024 | News Releases
MISSISSAUGA, CANADA, April 11, 2024 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces it will be attending and presenting at the Bloom Burton & Co. Healthcare Investor Conference (the...
by Microbix | Apr 2, 2024 | Media Featured Podcast
by microbix | Apr 1, 2024 | Media Featured Podcast
by Microbix | Apr 1, 2024 | Blog, News Releases
MISSISSAUGA, April 1, 2024 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF) (“Microbix®” or the Company”), a life sciences innovator and exporter, announces the voting results from the Annual and Special Meeting of Shareholders of the Company (the “Meeting”)...
by Microbix | Mar 15, 2024 | News Releases
Sequel Pharma Executes Agreement with CDMO for Drug Substance Production MISSISSAUGA, CANADA, March 14, 2024 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that its funding and...
by Microbix | Mar 11, 2024 | News Releases
HPV-related Tissue-Sample Mimics for QC of histology and PCR analyses MISSISSAUGA, CANADA, March 11, 2024 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that it is presenting results of...
by Microbix | Mar 7, 2024 | News Releases
ISO 13485:2016 Medical Devices Certification Attained TORONTO, December 24, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces that it has attained the ISO 13485:2016 Medical Devices...
by Microbix | Feb 14, 2024 | News Releases
Record Revenues of $8.4 million and Record Net Income of $2.5 million MISSISSAUGA, CANADA, February 14, 2024 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, reports results for its first...

by Microbix | Feb 11, 2024 | News Releases
Microbix will be hosting a virtual AGM on March 27, 2024, at 1:00 pm. The online link will allow shareholders to listen to the AGM and view a CEO presentation via Zoom. Shareholders without up-to-date computer access can listen-in via telephone by using the...
by Microbix | Feb 7, 2024 | News Releases
Results Release and Webinar Discussion on Morning of February 14, 2024 MISSISSAUGA, CANADA, February 7, 2024 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that it expects to file...
by Microbix | Feb 6, 2024 | News Releases
FLOQSwab-formatted QAP Supporting MDx Tests for H. pylori MISSISSAUGA, CANADA, February 6, 2024 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that it is presenting results of a Quality...
by Microbix | Jan 3, 2024 | News Releases
Over C$ 1 Million in One Shipment to a Leading Lab Accreditation Agency MISSISSAUGA, CANADA, January 3, 2024 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces it has sold and shipped over...
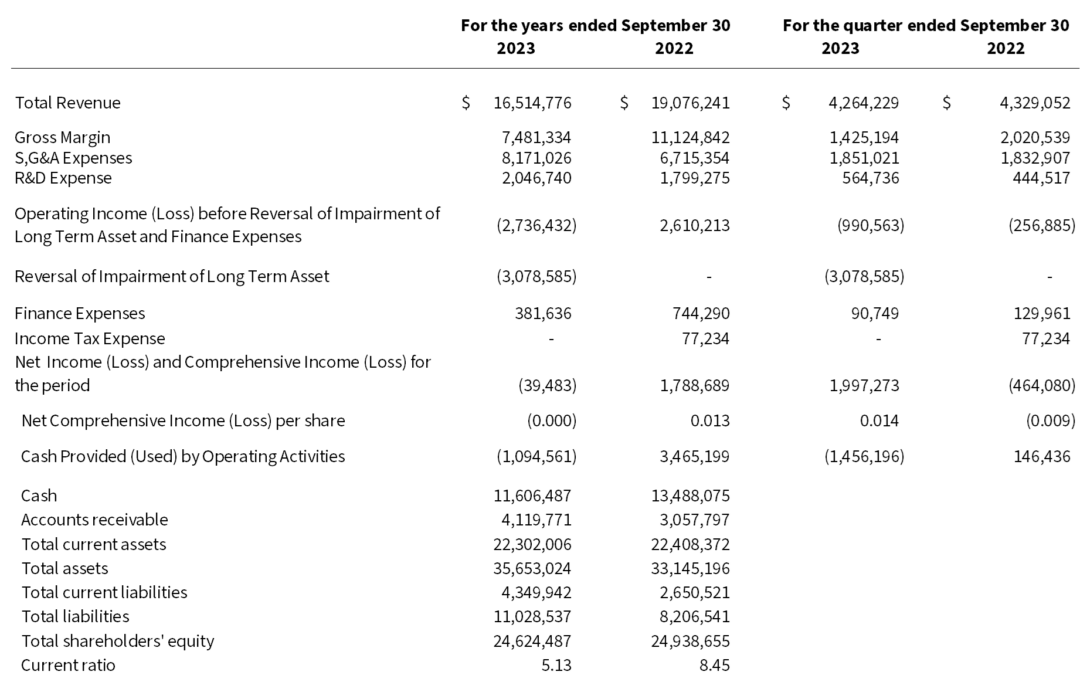
by Microbix | Dec 21, 2023 | News Releases
Full-Year Sales of $16.5 million and Net Loss of $0.04 million MISSISSAUGA, CANADA, December 21, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, reports results for its year and fourth...
by Microbix | Dec 14, 2023 | News Releases
Expanded Collaboration to Improve Syndromic Infectious Disease Testing MISSISSAUGA, CANADA & IRVINE, CALIFORNIA, December 14, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces a...
by Microbix | Dec 7, 2023 | News Releases
Quality Controls for Respiratory Viral, Pharyngitis, and Genital Ulcer Disease Panels MISSISSAUGA, CANADA & BIRMINGHAM, ALABAMA, December 7, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter,...
by Microbix | Dec 6, 2023 | News Releases
For Repurchase of up to 5% of its outstanding shares over 12 months MISSISSAUGA, CANADA, December 6, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces the initiation of a Normal...
by Microbix | Nov 17, 2023 | News Releases
Meetings with Growth-Oriented Investors, November 17 to 19, 2023 MISSISSAUGA, CANADA, November 17, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that it will be presenting to...
by Microbix | Nov 16, 2023 | News Releases
Sequel Pharma Reconfirms Kinlytic® urokinase Support for Catheter Clearance Indication MISSISSAUGA, CANADA, November 16, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces the...
by Microbix | Nov 10, 2023 | News Releases
Onboarding Tools and Quality Controls for Extended Genotype HPV Assays MISSISSAUGA, CANADA & TRIESTE, ITALY, November 10, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces a...
by Microbix | Nov 8, 2023 | Blog
Flu season is upon us, and with it comes the annual threat of influenza, respiratory syncytial virus (RSV), and more recently, the coronavirus, SAR-CoV-2. These viruses pose serious health risks to individuals around the world. The flu, in particular, can lead to a...
by Microbix | Oct 27, 2023 | News Releases
Distribution Partner Secures Contract Relating to Cervical Cancer Screening MISSISSAUGA, CANADA, October 27, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that its quality...
by Microbix | Oct 26, 2023 | Blog
In this episode of Diagnostics: Beyond the Lab we talk to two very distinguished guests, Christine Elliott, who served as the deputy premier of Ontario and the Minister of Health from 2018 to 2022, and Ivy Parks, President of Becton Dickinson (BD, for short) Canada,...
by Microbix | Oct 20, 2023 | Media Featured Podcast
by Microbix | Sep 29, 2023 | News Releases
Meetings with Growth-Oriented Investors, September 29 to October 1, 2023 MISSISSAUGA, CANADA, September 29, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that it will be...
by Microbix | Sep 27, 2023 | News Releases
Over C$ 1 Million in Orders from Leading Diagnostics Industry Test-Maker MISSISSAUGA, CANADA, September 27, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that it has secured record...
by Microbix | Sep 26, 2023 | Blog
In this episode we’re going to talk about current and emerging diagnostics technologies, how they’re viewed by government leaders, and how we can encourage their optimal use.
by Microbix | Sep 13, 2023 | News Releases
Microbix Honours the Passing of its Founder William J. Gastle – 1948 to 2023 MISSISSAUGA, CANADA, September 13, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, honours the passing of its...
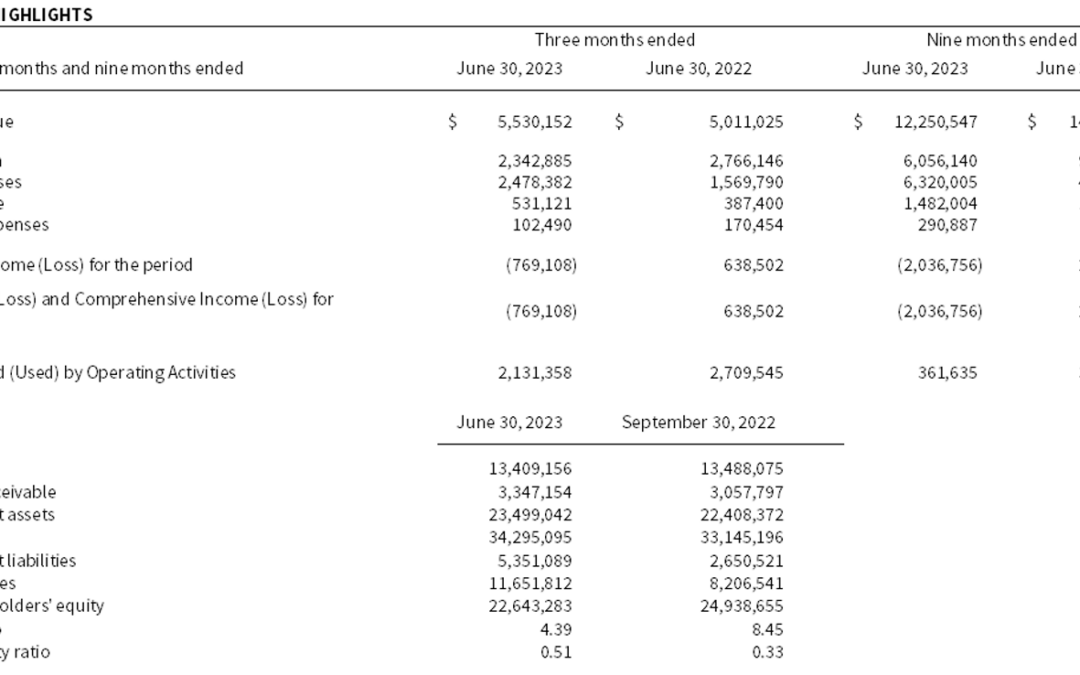
by Microbix | Aug 10, 2023 | News Releases
Strong Sales of $5.5 million alongside Drug Partnering Agreement MISSISSAUGA, August 10, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, reports results for its third quarter and YTD...
by Microbix | Aug 7, 2023 | News Releases
Achieves “Go-Live” with New ERP and eQMS Solutions MISSISSAUGA, CANADA, August 4, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces its successful adoption of software to upgrade,...
by Microbix | Jul 24, 2023 | News Releases
FLOQSwab-formatted QAPs Supporting Fourplex PCR Tests for Bacterial STIs MISSISSAUGA, CANADA, July 24, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that it is presenting results...
by Microbix | Jul 5, 2023 | News Releases
Distribution Partner Secures Contract Relating to Cervical Cancer Screening MISSISSAUGA, CANADA, July 5, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that its quality assessment...
by shitika | Jun 20, 2023 | Blog
So…you got your Respiratory infection test results back from the doctor. Should you trust them? Well, the answer is YES! Why? There is a sophisticated quality assurance ecosystem supporting diagnostic testing in most healthcare systems, ensuring reliable and accurate...
by Microbix | Jun 20, 2023 | Media Featured Poster Publication
https://microbix.com/wp-content/uploads/2022/05/ECCMID-2022-FINAL-March-29-2022.pdf
by Microbix | Jun 20, 2023 | News Releases
Innovative “EQA” Program to Qualify Labs for Genital Ulcer Disease Testing MISSISSAUGA, CANADA & HELSINKI, FINLAND – June 20, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter and Labquality...
by Microbix | Jun 18, 2023 | Media Featured Poster Publication
https://microbix.com//wp-content/AACC 2023 Final 21JUL2023.pdf
by Microbix | Jun 18, 2023 | Media Featured Poster Publication
https://microbix.com/wp-content/ASM 2023_FINAL COMMERCIAL COPY June 12 2023.pdf
by Pavel Zhelev | Jun 13, 2023 | News Releases
FLOQSwab-formatted QAPs supporting PCR Tests for Antibiotic-Resistant Pathogens MISSISSAUGA, CANADA, June 13, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter announces that it is presenting...
by Microbix | May 23, 2023 | Blog
In vitro diagnostics play a critical role in diagnosing and monitoring infectious diseases. Accurate and reliable test results are essential for guiding patient treatment and improving outcomes. However, with the increasing complexity of diagnostic tests, ensuring the...
by Pavel Zhelev | May 19, 2023 | News Releases
Applies for 12-month Term Extension of May 2021 Warrants MISSISSAUGA, CANADA, May 18, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces it has applied to the TSX to extend the...
by Microbix | May 16, 2023 | Blog
Point of care (POC) testing has revolutionized the way we diagnose and manage various infectious diseases, including sexually transmitted infections (STIs). The ability to obtain rapid, accurate results from POC tests has led to improved patient outcomes, reduced...
by Microbix | May 16, 2023 | News Releases
Kinlytic® urokinase to be developed for re-entry into U.S. market MISSISSAUGA, CANADA, May 16, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces execution of an agreement (“Agreement”)...
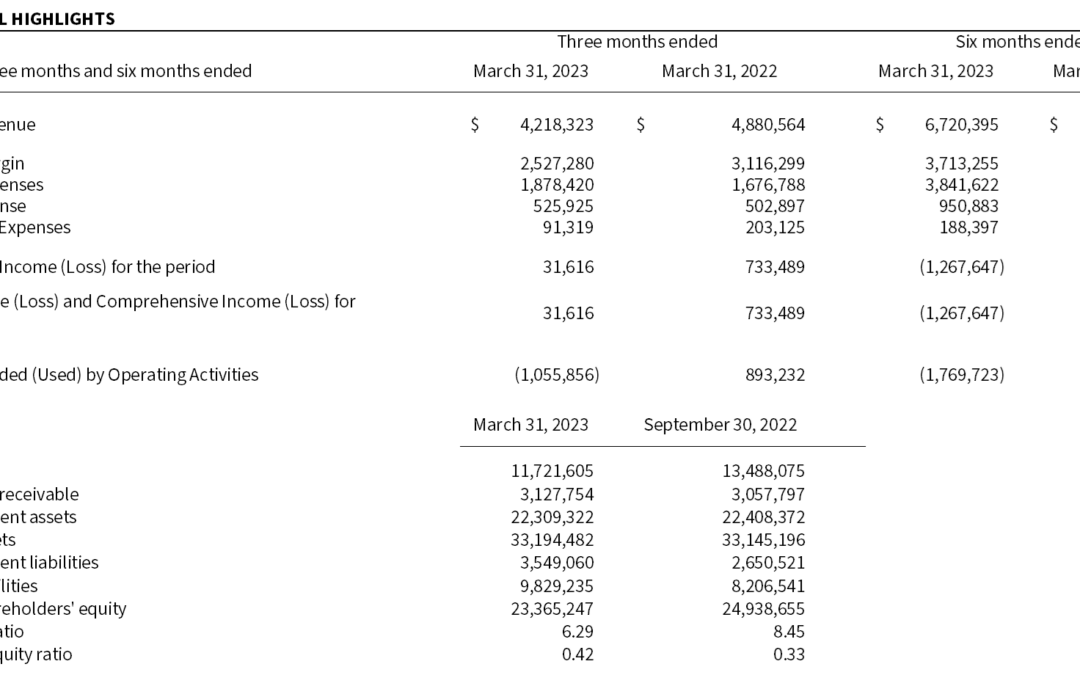
by Microbix | May 11, 2023 | News Releases
Sales of $4.2 million and Profitability MISSISSAUGA, May 11, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, reports results for its second quarter and first half of fiscal 2023 ended...
by Microbix | May 10, 2023 | Blog
In vitro diagnostics play a critical role in diagnosing and monitoring infectious diseases. Accurate and reliable test results are essential for guiding patient treatment and improving outcomes. However, with the increasing complexity of diagnostic tests, ensuring the...
by Microbix | May 10, 2023 | Blog
Point of care (POC) testing has revolutionized the way we diagnose and manage various infectious diseases, including sexually transmitted infections (STIs). The ability to obtain rapid, accurate results from POC tests has led to improved patient outcomes, reduced...
by Microbix | May 9, 2023 | Blog
Trichomonas vaginalis, also known as trich, is a sexually transmitted infection (STI) caused by a parasitic protozoan. According to WHO more than 1 million new STIs are acquired every day and approximately 357 million people contract STIs each year. Despite its...
by Microbix | May 2, 2023 | Blog
Did you know that sexually transmitted infections (STIs) are shockingly common? Millions of cases are diagnosed every year, with young people and men who have sex with men being particularly at risk. And the scariest part? Many people don’t even know they have...
by Microbix | Apr 25, 2023 | Blog
Antimicrobial resistance (AMR) is a serious global health issue that is affecting millions of people every year. It refers to the ability of microorganisms to resist the effects of antimicrobial drugs, such as antibiotics. This phenomenon has been on the rise in...
by Microbix | Apr 24, 2023 | Media Featured Podcast
with Daniel Taylor from Oneworld Accuracy, Dannielle Casey from American Proficiency Institute, and Heidi Berghall from Labquality
by Pavel Zhelev | Apr 20, 2023 | News Releases
MISSISSAUGA, CANADA, April 20, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces it will be attending and presenting at the Bloom Burton & Co. Healthcare Investor Conference (the...
by Microbix | Apr 18, 2023 | Blog
STIs: A public health issue Sexually transmitted infections (STIs) are a significant public health issue, with millions of new cases diagnosed each year. Accurate and timely diagnosis is crucial to prevent the spread of these infections and ensure that patients...
by Microbix | Apr 10, 2023 | Blog
Syphilis is a sexually transmitted infection (STI) caused by the bacterium Treponema pallidum. The infection can cause serious health problems if left untreated, including blindness, deafness, and even death. In Canada, syphilis rates have been on the rise in recent...
by Microbix | Apr 3, 2023 | Blog
Sexually transmitted infections (STIs) affect millions of people globally, yet there remains a significant amount of stigma around these infections. This stigma prevents people from discussing their STI status, leading to negative health outcomes and further spread of...
by Pavel Zhelev | Mar 30, 2023 | News Releases
MISSISSAUGA, March 30, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF)( “Microbix®” or the Company”), a life sciences innovator, manufacturer, and exporter, announces the voting results from the Annual and Special Meeting of Shareholders of the Company...
by Microbix | Mar 29, 2023 | Blog
Get to know these outstanding guest speakers: Daniel Taylor from 1WA, Danielle Casey from API, and Heidi Berghäll from Labquality! Discover their expertise and insights on healthcare, technology, and more.
by Microbix | Mar 29, 2023 | Blog
Laboratory testing requires accuracy and reliability for patient care. External Quality Assessment (EQA) is an effective tool to ensure testing accuracy and reliability. EQA compares laboratory test results with peer groups or reference labs worldwide. This article...
by shitika | Mar 22, 2023 | Blog, Media Features News Releases, News Releases
Remote Access to the AGM Provided to Avoid Physical Attendance MISSISSAUGA, CANADA, March 22, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator making critical ingredients that enable the production of clinical...
by Microbix | Mar 20, 2023 | Blog
Lessons from the COVID-19 Pandemic The COVID-19 pandemic has taught us that pandemics can be incredibly disruptive to our society, economy, and daily lives. In order to be better prepared for future pandemics, the whole industry has invested in scaling up the...
by Pavel Zhelev | Mar 20, 2023 | News Releases
$ 1.68 Million in Funding for Capabilities and Capacity Expansions MISSISSAUGA, CANADA, March 20, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that it is undertaking further...
by shitika | Mar 7, 2023 | Blog
The Role of Diagnostics in HPV Screening The Importance of HPV Screening Human papillomavirus (HPV) is a common sexually transmitted infection that is linked to the development of cervical cancer. The primary screening for HPV infection is an important tool in the...
by Pavel Zhelev | Mar 1, 2023 | News Releases
QAPs used in Diagnostic Testing for STIs in Remote Communities MISSISSAUGA, CANADA, March 1, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that its Quality Assessment Products...
by shitika | Feb 27, 2023 | Blog, Media Features News Releases, News Releases
Reading Time: 4 minutes Using Shareholder-Approved Plan to Incentivize and Retain MISSISSAUGA, CANADA, February 27, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer and exporter, announces the issuance...
by Microbix | Feb 21, 2023 | Blog
The Antimicrobial Resistance (AMR) crisis and how we can overcome it Antimicrobial Resistance (AMR) Antimicrobial resistance or AMR occurs when microorganisms build resistance to an antibiotic drug. With AMR on the rise, the World Health Organization has deemed this a...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Pavel Zhelev | Feb 9, 2023 | News Releases
Sales of $2.5 million, Net Loss of $1.3 million MISSISSAUGA, February 9, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, reports results for its first quarter ended December 31, 2022...
by Microbix | Feb 8, 2023 | Media Featured Upcoming Events, Upcoming Events
Muskoka Capital Event September 23-25 1050 Paignton House Road Minett, ON P0B 1G0 Canada
by Pavel Zhelev | Feb 6, 2023 | News Releases
QAPs for HPV Test Verification within Cervical Cancer Screening Program MISSISSAUGA, CANADA, February 6, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that it will be presenting...
by Microbix | Feb 2, 2023 | Media Featured Upcoming Events, Media Features News Releases
https://microbix.com/upcoming-events-2/
by Pavel Zhelev | Jan 30, 2023 | News Releases
Driven by Customer Requests, Mpox Test Control Now Available MISSISSAUGA, CANADA, January 30, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that it is launching a new quality...
by Microbix | Jan 27, 2023 | Blog
Here are all of Microbix’s upcoming events including conferences, trade shows, presentations, and more! Make sure to check regularly to stay up to date with the latest news from us!
by Microbix | Jan 13, 2023 | Blog
In this episode of Diagnostics: Beyond the Lab we talk to Giorgio Triva, CEO and Strategic Project Manager of Copan Group, based in Italy, about what his company and the work it has done to become a global leader in sample collection, transport and test accuracy for...
by Microbix | Jan 10, 2023 | Blog
In this episode of Diagnostic: Beyond the lab, we talk to Colin Denver, CEO of SpeeDX about antimicrobial resistance and using diagnostics to target treatment.
by Microbix | Jan 5, 2023 | Blog
Diagnostics: Beyond the Lab In this episode we discuss the Human papillomavirus or HPV, vaccines, testing and cervical cancer. What is HPV and what is the way forward for testing?Joining us for this discussion is Larry Vaughan, director scientific affairs integrated...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Pavel Zhelev | Dec 22, 2022 | Blog, News Releases
Record Sales of $19.1 million, Net Earnings of $1.8 million MISSISSAUGA, December 22, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, reports results for its year and fourth quarter...
by E V | Nov 28, 2022 | Media Featured Investor Info
https://microbix.com/wp-content/uploads/2022/11/MBX-Corporate-Presentation-2022-11-25-3.pdf
by E V | Nov 28, 2022 | Media Featured Investor Info
Download the Microbix Corporate Presentation – November 2022
by Microbix | Nov 11, 2022 | Media Featured Financial Reports
https://microbix.com/wp-content/uploads/2022/05/Microbix-QR_2-2022-v3-FINAL-PRINT.pdf
by Microbix | Nov 10, 2022 | Media Featured White Paper
https://microbix.com/wp-content/QAP/White%20Papers/HPV_White%20Paper.pdf
by E V | Nov 10, 2022 | Media Featured Financial Reports
https://microbix.com/financial-reports/
by Microbix | Nov 9, 2022 | Media Featured White Paper
https://microbix.com/wp-content/QAP/White%20Papers/REDx_SARS%20Molecular_WhitePaper.pdf
by Microbix | Nov 9, 2022 | Media Featured Financial Reports
https://microbix.com/wp-content/uploads/2022/08/Microbix-QR_3-2022-v3-FINAL-PRINT.pdf
by Microbix | Nov 8, 2022 | Media Featured White Paper
https://microbix.com/wp-content/QAP/White%20Papers/REDx_WhitePaper_SARS_Ag-swab.pdf
by Microbix | Nov 8, 2022 | Media Featured Poster Publication
**https://microbix.com/wp-content/QAP/Poster%20Publications/HPV/HPV_Labquality_2020.pdf
by Microbix | Nov 8, 2022 | Media Featured Poster Publication
https://microbix.com/wp-content/QAP/Poster%20Publications/HPV/EUROGIN2021_HPV.pdf
by Microbix | Nov 7, 2022 | Media Featured Poster Publication
https://microbix.com/wp-content/uploads/2022/05/EUROGIN-2022_FINAL-March-29-2022.pdf
by Microbix | Nov 6, 2022 | Media Featured White Paper
https://microbix.com/wp-content/uploads/2022/05/ECCMID-2022-FINAL-March-29-2022.pdf
by Microbix | Nov 6, 2022 | Media Featured Poster Publication
https://microbix.com/wp-content/QAP/Poster%20Publications/MG/AACC2020%20MG.pdf
by Microbix | Nov 5, 2022 | Media Featured Poster Publication
https://microbix.com/wp-content/uploads/2022/06/AACC-PreAnalytical-conference-June-2022_revised-FINAL.pdf
by Microbix | Nov 5, 2022 | Media Featured Financial Reports
https://microbix.com/wp-content/uploads/2022/02/Microbix-QR_1-2022-PRINT-VERSION.pdf
by Microbix | Nov 4, 2022 | Media Featured Poster Publication
https://microbix.com/wp-content/QAP/Poster%20Publications/SARS/AACC%202021%20VOC.pdf
by Microbix | Nov 4, 2022 | Media Featured Podcast
by Microbix | Nov 3, 2022 | Media Featured Poster Publication
https://microbix.com/wp-content/QAP/Poster%20Publications/SARS/ECCMID2021%20SARS%20antigen.pdf
by Microbix | Nov 2, 2022 | Media Featured Poster Publication
https://microbix.com/wp-content/QAP/Poster%20Publications/SARS/ECCMID2021_SARS%20Ab.pdf
by Microbix | Nov 1, 2022 | Media Featured Poster Publication
https://microbix.com/wp-content/QAP/Poster%20Publications/SARS/ECCVID%202020%20SARS.pdf
by Microbix | Nov 1, 2022 | Media Featured Podcast
by Microbix | Oct 31, 2022 | Media Featured Investor Info
Cameron Groome, CEO of Microbix (TSX: MBX), provides an update on operations and opportunities amid the COVID-19...
by Microbix | Oct 31, 2022 | Media Featured Investor Info
https://microbix.com/wp-content/MBX%20-%20AGM%20PPT%20-%20V4%20-%202022-03-30.pdf
by Microbix | Oct 31, 2022 | Media Featured Investor Info
https://microbix.com/wp-content/MBX%20Corporate%20Presentation%20-%202022-9-19.pdf
by Microbix | Oct 31, 2022 | Media Featured Investor Info
https://microbix.com/wp-content/MBX%20Corporate%20Presentation%20-%202022-9-19.pdf
by Microbix | Oct 31, 2022 | Media Featured Investor Info
https://microbix.com/wp-content/uploads/2022/10/MBX-Corporate-Presentation-2022-10-24.pdf
by Microbix | Oct 24, 2022 | Media Featured Global
https://microbix.com/wp-content/QAP/Poster%20Publications/HPV/HPV_Labquality_2020.pdf
by Microbix | Oct 24, 2022 | Media Featured Global
https://microbix.com/wp-content/QAP/Poster%20Publications/HPV/EUROGIN2021_HPV.pdf
by Microbix | Oct 18, 2022 | Media Featured Global
https://microbix.com/wp-content/QAP/Poster%20Publications/FluRSV/EMMD2019%20Flu_RSV.pdf
by Pavel Zhelev | Oct 6, 2022 | News Releases
MSwab® & PROCEEDx™FLOQ® Supporting Self-Collection for HPV Screening Programs MISSISSAUGA, CANADA, October 6, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that, on behalf of...
by Pavel Zhelev | Sep 28, 2022 | Media Features News Releases, News Releases
Microbix to Repurchase up to 5% of its outstanding shares over 12 months MISSISSAUGA, CANADA, September 28, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces the initiation of a Normal...
by E V | Sep 22, 2022 | Media Features News Releases
https://microbix.com/wp-content/uploads/2023/02/MBX-NR-Q1-2023-Results-V5-CLEAN-2023-1-31.pdf
by Pavel Zhelev | Sep 21, 2022 | Media Features News Releases, News Releases
Meetings with Growth-Oriented Investors, September 23-25, 2022 MISSISSAUGA, CANADA, September 21, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces that it will be presenting to...
by Pavel Zhelev | Aug 12, 2022 | Media Features News Releases, News Releases
Q3 Sales of $5.0 million, Q3 Net Earnings of $0.6 million MISSISSAUGA, August 11, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, reports results for its third quarter and year-to-date...
by Pavel Zhelev | Aug 10, 2022 | Media Features News Releases, News Releases
Microbix QAPs to Support Molecular & Antigen Test Platforms for Multiple Diseases MISSISSAUGA, CANADA, August 10, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces the execution of...
by Pavel Zhelev | Jul 26, 2022 | News Releases
Quality Control Materials for Genital Ulcer Disease Molecular Assays MISSISSAUGA, CANADA, July 26, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces it is presenting about the utility...
by Microbix | Jul 25, 2022 | Media Featured Upcoming Events, Upcoming Events
Florida Capital Event November 11-13 3555 S Ocean Dr Hollywood, FL 33019 USA
by Microbix | Jun 22, 2022 | Blog
ABOUT MICROBIX Microbix: Who we are? Microbix Biosystems Inc. develops and commercializes proprietary biological and technological solutions for human health and well-being. We manufacture a wide range of biological materials for the global diagnostics industry,...
by Pavel Zhelev | Jun 3, 2022 | News Releases
Usage of QAPs to Validate Whole Workflow Quality Control of Testing MISSISSAUGA, CANADA, June 3, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces it is presenting about the utility of...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Pavel Zhelev | May 12, 2022 | News Releases
Record Q2 Sales of $4.9 million, Q2 Net Earnings of $0.7 million MISSISSAUGA, May 12, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, reports results for its second quarter and first...
by Pavel Zhelev | Apr 29, 2022 | News Releases
QAPs Supporting Molecular Diagnostic Tests for Dominant COVID-variant MISSISSAUGA, CANADA, April 29, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces it will present performance...
by Pavel Zhelev | Apr 28, 2022 | News Releases
Healthcare Investor Conference in Toronto on May 2 & 3, 2022 MISSISSAUGA, CANADA, April 28, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces it will be attending and presenting at...
by Pavel Zhelev | Apr 19, 2022 | News Releases
QAPs Supporting Tests That Simultaneously Detect Multiple Respiratory Viruses MISSISSAUGA, CANADA, April 19, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, announces it will be presenting...
by Pavel Zhelev | Apr 8, 2022 | News Releases
QAPs Supporting Screening-Tests for High-Risk Human Papilloma Viruses MISSISSAUGA, CANADA, April 8, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces it will present performance results of its...
by Pavel Zhelev | Mar 31, 2022 | News Releases
Microbix Announces Annual and Special Meeting Voting Results MISSISSAUGA, CANADA, March 31, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces the voting results from its Annual and Special...
by Pavel Zhelev | Mar 29, 2022 | News Releases
Implementing MasterControl® and NetSuite® Solutions to Enable Rapid Growth MISSISSAUGA, CANADA, March 29, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces investment in programs to upgrade, digitize...
by Pavel Zhelev | Mar 8, 2022 | Events
Once again, due to COVID -19 Pandemic, Microbix will be hosting a virtual AGM on March 30, 2022 at 1:00 pm. The online link will allow shareholders to listen to the AGM and view a CEO presentation via Zoom. Shareholders without up-to-date computer access can...
by Microbix | Mar 3, 2022 | Events
The time has come again for the Canadian annual meeting in infectious diseases and diagnostic microbiology. The AMMI Canada – CACMID Annual Conference is returning in a hybrid format: in-person in Vancouver, BC, and online via our virtual platform. The AMMI Canada –...
by Microbix | Mar 3, 2022 | Events
The EUROGIN 2022 Organizing Committee is happy to welcome the HPV community to gather again in person in Düsseldorf on April 10-12, 2022, after a difficult period owing to the COVID-19 pandemic. EUROGIN Conference 2022 is the first large international in-person...
by Microbix | Mar 3, 2022 | Upcoming Events
Labquality Days offers an extensive and diverse exhibition on top of congress. The exhibition offers companies excellent marketing and networking opportunities. Labquality Days congress gathers together medical laboratory and quality management professionals and...
by Microbix | Mar 3, 2022 | Upcoming Events
ECCMID, as the world’s premier Clinical Microbiology & Infectious Diseases event, brings together experts from many fields to present their latest findings, guidelines and experiences to an audience of over 14,000 colleagues. This year, the congress will feature a...
by Pavel Zhelev | Feb 24, 2022 | News Releases
Using Shareholder-Approved Plan to Incentivize and Retain MISSISSAUGA, CANADA, February 24, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces the issuance of stock options under its...
by Pavel Zhelev | Feb 22, 2022 | News Releases
For Appointment of Jennifer Stewart, Accomplished Business Leader MISSISSAUGA, CANADA, February 22, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces that Ms. Jennifer Stewart, an accomplished leader...
by Pavel Zhelev | Feb 17, 2022 | News Releases
Meetings with Growth-Oriented Investors, February 18-20, 2022 MISSISSAUGA, CANADA, February 17, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces that it will be presenting to equity investors...
by Pavel Zhelev | Feb 15, 2022 | News Releases
Expansion of Capacity for Manufacturing of QAPs & DxTM MISSISSAUGA, CANADA, February 15, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces progress with expanding manufacturing capacity of its...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Pavel Zhelev | Feb 10, 2022 | News Releases
Record Q1 Sales of $4.9 million, Record Q1 Net Earnings of $0.9 million MISSISSAUGA, February 10, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, reports results for its first quarter ended December...
by Pavel Zhelev | Feb 1, 2022 | News Releases
Helping Ensure Workflow Accuracy of Tests for Respiratory Viruses MISSISSAUGA, CANADA, February 1, 2022 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces the expansion of its portfolio of Quality...
by Pavel Zhelev | Dec 24, 2021 | News Releases
Follow-on Order from Government of Ontario Procurement Authorities MISSISSAUGA, CANADA, December 24, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces receipt of a follow-on order of C$ 4.7 million...
by Pavel Zhelev | Dec 23, 2021 | News Releases
Record Sales of $18.6 million, Record Net Earnings of $3.2 million MISSISSAUGA, December 23, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, reports results for its year and fourth quarter ended...
by Pavel Zhelev | Dec 20, 2021 | News Releases
Helping Ensure Workflow Accuracy of Tests for COVID, Flu A, Flu B, and RSV MISSISSAUGA, CANADA, December 20, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces the expansion of its portfolio of...
by Pavel Zhelev | Nov 17, 2021 | News Releases
Helping Ensure Workflow Accuracy of PCR Tests for COVID “Variants of Concern” MISSISSAUGA, CANADA, November 17, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces the expansion of its portfolio of...
by Pavel Zhelev | Oct 27, 2021 | News Releases
Further Capital to Assist with Increasing Capacity and Efficiency MISSISSAUGA, CANADA, October 27, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces that it has received C$ 2.7 million from the...

by Pavel Zhelev | Oct 6, 2021 | Events
Advanced SARS-CoV-2 Variants of Concern (VOC) whole genome materials for use as verification, external quality assessment (EQA) and prospective quality control samples Click below to download the poster:
by Pavel Zhelev | Oct 4, 2021 | News Releases
$1.3 million balance repaid, improving cash flow and resulting in non-cash charge MISSISSAUGA, CANADA, October 4, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces that it has completed the early...
by Pavel Zhelev | Sep 27, 2021 | News Releases
QAPs to Support Testing for COVID Viral Variants MISSISSAUGA, CANADA, September 27, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces that it has been invited to present about the performance of its...
by Pavel Zhelev | Sep 23, 2021 | Events
Meetings with Growth-Oriented Investors, September 24-26, 2021 MISSISSAUGA, September 23, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces that it will be presenting to investors at the...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Pavel Zhelev | Aug 12, 2021 | News Releases
Record Sales of $5.5 million, Record Net Earnings of $1.5 million MISSISSAUGA, August 12, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, reports results for its third quarter and nine months of fiscal...
by Pavel Zhelev | Aug 3, 2021 | News Releases
Trading Upgrade to OTCQX Best Market and Torrey Hills Capital Engaged MISSISSAUGA, ON, August 3, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator and exporter, announces that it has undertaken two actions to improve its...
by Pavel Zhelev | Jul 16, 2021 | Events
Two Posters Reporting on Cross-Platform Utility of Antigen & Serological QAPs™ Novel neutralizing anti-S1 SARS-CoV-2 human monoclonal antibody formulation for use as a cross-platform EQA sample and prospective Quality Controls Advanced SARS-CoV-2 nucleocapsid...
by Pavel Zhelev | Jul 8, 2021 | News Releases
Two Abstracts Reporting on Cross-Platform Utility of Antigen & Serological QAPs™ MISSISSAUGA, CANADA, July 8, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces that it has been invited to present...
by Pavel Zhelev | Jul 7, 2021 | News Releases
Appointment of Thomas Scientific as U.S. Distributor MISSISSAUGA, July 7, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces the appointment of Thomas Scientific LLC as distributor of Microbix’s...
by Pavel Zhelev | Jun 3, 2021 | News Releases
Leasing and Equipping 10,000 Square Foot Building Adjacent to Current Sites MISSISSAUGA, June 3, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces that it has secured a third manufacturing site in...
by Pavel Zhelev | May 31, 2021 | News Releases
Appointment of SDT Molecular of Singapore MISSISSAUGA, May 31, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces the appointment of SDT Molecular Pte Ltd (SDT) as distributor of Microbix’s quality...
by Pavel Zhelev | May 19, 2021 | News Releases
$6.9 Million of Gross Proceeds Received – NOT FOR DISSEMINATION IN THE UNITED STATES OR THROUGH U.S. NEWSWIRE SERVICES – MISSISSAUGA, May 19, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter,...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Pavel Zhelev | May 13, 2021 | News Releases
Record Q2 Sales of $4.35 million, Record Net Earnings of $0.8 million MISSISSAUGA, May 13, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, reports results for its second quarter and first half of...
by Pavel Zhelev | Apr 28, 2021 | News Releases
$5.0 Million Bought-Deal Public Offering and $1.0 Million Private Placement – NOT FOR DISSEMINATION IN THE UNITED STATES OR THROUGH U.S. NEWSWIRE SERVICES – MISSISSAUGA, April 28, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life...
by Pavel Zhelev | Apr 26, 2021 | News Releases
Focus on Diagnostics and Controls for Anti-Microbial Resistant Infections MISSISSAUGA, CANADA and EVELEIGH, AUSTRALIA April 26, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, and SpeeDx Pty. Ltd. (SpeedDx),...
by Pavel Zhelev | Apr 23, 2021 | News Releases
An Initial Order From Government Of Ontario Procurement Authorities MISSISSAUGA, April 23, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces that it has received an initial order of CDN$ 4.25 million...
by Pavel Zhelev | Apr 19, 2021 | Events
Virtual Healthcare Investor Conference on April 20 & 21 MISSISSAUGA, April 19, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces that its CEO, Cameron Groome, has been invited to present at...
by Pavel Zhelev | Mar 31, 2021 | News Releases
Reading Time: 3 minutes MISSISSAUGA, March 31, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF)( “Microbix®” or the Company”), a life sciences innovator and exporter, announces the voting results from the Annual and Special Meeting of Shareholders of the...
by Pavel Zhelev | Mar 30, 2021 | Events
Once again, due to COVID -19 Pandemic, Microbix will be hosting a virtual AGM on March 30, 2021 at 1:00 pm. The online link will allow shareholders to listen to the AGM and view a CEO presentation via Zoom. Shareholders without up-to-date computer access can...
by Pavel Zhelev | Mar 15, 2021 | News Releases
Focus on Supporting Canadian Labs with Variant-related Assays and QAPs MISSISSAUGA & TORONTO, March 15, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, and Seegene Canada Inc. (Seegene®), a provider of...
by Pavel Zhelev | Mar 11, 2021 | News Releases
REDxFLOQ SARS-CoV-2 Antigen as IVD Controls in Canada, the EU, and the U.S. MISSISSAUGA, March 11, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces the availability of more of its Quality Assessment...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Mar 3, 2021 | Blog
During the first peak of the coronavirus pandemic, it became apparent that the processing of the standard COVID-19 test using nasal swab samples, while considered a gold standard, was slow, taking over 24 hours to give results in most cases. As demand for quicker test...
by Pavel Zhelev | Feb 18, 2021 | News Releases
Reading Time: 4 minutes Using Shareholder-Approved Plan to Incentivize and Retain MISSISSAUGA, February 18, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces the issuance of stock options under...
by Pavel Zhelev | Feb 16, 2021 | News Releases
Oneworld Accuracy as a Non-Exclusive Distributor for Africa and Other Territories MISSISSAUGA, February 16, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces an agreement (Agreement) with Oneworld...
by Pavel Zhelev | Feb 11, 2021 | News Releases
Record Q1 Sales, With Nearly 3,600% Year-Over-Year QAPs Growth MISSISSAUGA, February 11, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, reports results for the first quarter of its fiscal 2021, the...
by Pavel Zhelev | Feb 9, 2021 | News Releases
Initial Production of 50,000 Vials Entirely Sold MISSISSAUGA, February 9, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, is pleased to announce material first sales of its viral transport medium...
by Pavel Zhelev | Jan 28, 2021 | News Releases
Initial Capacity of 50,000 Vials/Week under Health Canada Regulation MISSISSAUGA, January 28, 2021 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, is pleased to announce the commercial availability of its viral...
by Pavel Zhelev | Dec 21, 2020 | News Releases
For FLOQ devices & QAPs Co-branding, IP licensing, Co-marketing, Purchase, and Supply MISSISSAUGA, CANADA and BRESCIA, ITALY, December 21, 2020 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, and Copan Italia...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Pavel Zhelev | Dec 18, 2020 | News Releases
Full-Year Sales of $10.5 Million, QAPs Sales Grow by 41% MISSISSAUGA, December 18, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), a life sciences innovator making critical ingredients that enable the production of clinical diagnostics and creating...
by Pavel Zhelev | Dec 15, 2020 | News Releases
Reporting on Test-Workflow Controls for Important Sexually-Transmitted Infections MISSISSAUGA, CANADA, December 15, 2020 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces it has been invited to present...
by Pavel Zhelev | Dec 7, 2020 | News Releases
Appointment of Anthony J. Giovinazzo, Accomplished Life Sciences Leader MISSISSAUGA, CANADA, December 7, 2020 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces that Mr. Anthony J. Giovinazzo, an...
by Microbix | Oct 29, 2020 | News Releases
Sales Of QAPs™ Kits To Help Qualify Multi-Pathogen Respiratory Test Systems MISSISSAUGA, CANADA, October 29, 2020 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces the availability and initial material...
by Microbix | Oct 20, 2020 | News Releases
QAPs™ To Support Accuracy Of COVID-19 Antigen-Test Workflows MISSISSAUGA, CANADA, October 20, 2020 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, announces its creation, external verification, initial...
by Microbix | Oct 13, 2020 | News Releases
Grant Of $1.45 Million For Ontario-Based Production Of Critical Test Reagents MISSISSAUGA, October 13, 2020 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), a life sciences innovator and exporter, is pleased to announce the execution of a grant...
by Microbix | Sep 22, 2020 | News Releases
Abstract Reports Multi-Platform Utility Of IVD SARS CoV-2 Lab Workflow Control MISSISSAUGA, CANADA, September 22, 2020 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), an award-winning life sciences innovator and exporter, announces it is has been...
by Microbix | Sep 17, 2020 | News Releases
Applies For 12-Month Term Extension Of October 2015 And October 2017 Warrants MISSISSAUGA, CANADA, September 17, 2020 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), an award-winning life sciences innovator and exporter, announces it has applied...
by Microbix | Sep 15, 2020 | News Releases
William J. Gastle Retiring, Martin Marino Nominated To Become Independent Chairman MISSISSAUGA, CANADA, September 15, 2020 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), an award-winning life sciences innovator and exporter, announces that its founder...
by Microbix | Sep 10, 2020 | News Releases
Enables Use of COVID-19 and HPV Products by Australian Clinical Labs MISSISSAUGA, CANADA, September 10, 2020 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), an award-winning life sciences innovator and exporter, is pleased to announce attainment of...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Aug 14, 2020 | News Releases
Year-Over-Year QAPs Sales Growth of 141% for Q3 MISSISSAUGA, August 14, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, reports results for its third quarter and year to date fiscal 2020 (Q3 and YTD)...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Jul 21, 2020 | Blog
Curious to see how we perform a COVID test? The video shows the laboratory steps involved in testing four patient samples along with one Control. The reagents and procedures are for the “CDC test” for SARS-CoV-2. Still, there is no difference in processing these...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Jul 14, 2020 | News Releases
OTC Markets Listing and DTC Electronic Clearance Eligibility MISSISSAUGA, ON, CANADA, July 14, 2020 – Microbix Biosystems Inc. (TSX: MBX, OTCQB: MBXBF, Microbix®), an award-winning life sciences innovator and exporter, announces that it has completed two processes to...
by microbix | Jul 7, 2020 | Events
Please note, due to the global circumstances, this year’s Microbix Annual General Meeting will be held as a virtual meeting. Please stay tuned for link and instructions.
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Jun 5, 2020 | News Releases
Enables Usage of SARS-CoV-2 Controls by Clinical Labs across Europe MISSISSAUGA, CANADA, June 5, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, is pleased to announce the attainment of European Union “CE...
by Microbix | Jun 4, 2020 | News Releases
Appointment of Medical Supply Company of Ireland MISSISSAUGA, March 24, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), a life sciences innovator making critical ingredients that enable the production of clinical diagnostics and creating medical devices that...
by Microbix | Jun 2, 2020 | News Releases
Instrument-Specific Controls to Support Rapid Tests for Viral Respiratory Pathogens MISSISSAUGA, June 1, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, announces that it has been engaged to develop and...
by Microbix | May 27, 2020 | News Releases
Build-out to Support Tenfold Increase Now Underway MISSISSAUGA, CANADA, May 27, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, announces that it has begun a build-out of its second facility that is...
by Microbix | May 19, 2020 | News Releases
Six Units in Ongoing Use for Foreseeable Future, Seventh Ordered MISSISSAUGA, CANADA, May 19, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, announces that it has achieved full utilization of the six...
by Microbix | May 15, 2020 | News Releases
Appointment of Labquality Oy of Finland for 7 Countries MISSISSAUGA, April 27, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, announces the appointment of Labquality Oy (Labquality) as a distributor of its...
by Microbix | May 14, 2020 | News Releases
Year-Over-Year Sales Growth of 28% in First-Half TORONTO, May 14, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, reports financial results for its second quarter of fiscal 2018, the three-month period...
by Microbix | May 14, 2020 | News Releases
Timing of Product Shipments Affects Results MISSISSAUGA, May 14, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, reports results for its second quarter and first half of fiscal 2020 (“Q2” and “H1”)...
by Microbix | May 12, 2020 | News Releases
Appointment of D.I.D. S.p.A. of Milan for Italy MISSISSAUGA, May 12, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, announces the appointment of Diagnostic International Distribution S.p.A. of Milan...
by Microbix | May 7, 2020 | News Releases
Registration Enables Immediate Usage of SARS-CoV-2 Controls by U.S. Clinical Labs MISSISSAUGA, CANADA, May 7, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, is pleased to announce U.S. availability of its...
by Microbix | May 6, 2020 | News Releases
SARS-CoV-2 Controls for Canadian Labs in Swab and Vial Formats MISSISSAUGA, CANADA, May 6, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, announces that it has begun providing its REDx™FLOQ® SARS-CoV-2...
by Microbix | Apr 27, 2020 | News Releases
Appointment of R-Biopharm AG for 11 Countries MISSISSAUGA, May 15, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, announces the appointment of R-Biopharm AG (R-Biopharm) as distributor of its Quality...
by Microbix | Apr 21, 2020 | Events
Presentation and Panel Participation at Adelaide Capital Event on April 23 MISSISSAUGA, CANADA, April 21, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, announces that it will be participating in the...
by Microbix | Apr 21, 2020 | News Releases
Enables Immediate Usage of SARS-CoV-2 Controls by Clinical Labs across Canada MISSISSAUGA, CANADA, April 21, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), a life sciences innovator and exporter, announces that it has attained medical devices establishment...
by Microbix | Apr 14, 2020 | Blog
Cameron Groome – Mississauga Company Contributes to COVID-19 Solution –...
by Microbix | Apr 13, 2020 | Blog
by Microbix | Apr 9, 2020 | News Releases
Laboratory External Quality Assessment, Proficiency Testing, & Accreditation MISSISSAUGA, ONTARIO and VANCOUVER, BC, CANADA, April 9, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®) and Oneworld Accuracy (1WA) announce a strategic collaboration to provide a...
by Microbix | Apr 1, 2020 | News Releases
TORONTO, April 2, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces the voting results from the Annual and Special Meeting of Shareholders (the “Meeting”) that was held on March 28, 2018. At...
by Microbix | Mar 30, 2020 | News Releases
Whole-genome QMS Support Tool on COPAN® FLOQSwabs® MISSISSAUGA, CANADA, March 30, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®) announces the creation, verification, and imminent commercial availability of a quality assessment product (QAP™) to help ensure the...
by Microbix | Mar 25, 2020 | News Releases
Remote Access to the AGM Provided to Avoid Physical Attendance MISSISSAUGA, March 25, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), a life sciences innovator making critical ingredients that enable the production of clinical diagnostics and creating...
by Microbix | Mar 24, 2020 | News Releases
Appointment of Alpha-Tec Systems, Inc. for the United States MISSISSAUGA, June 4, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an award-winning life sciences innovator and exporter, announces the appointment of Alpha-Tec Systems, Inc. (Alpha-Tec), a Calibre...
by Microbix | Feb 25, 2020 | News Releases
Customer Validation of New Manufacturing Process Completed TORONTO: September 13, 2017 – Microbix Biosystems Inc. (TSX: MBX), a developer and marketer of biological products and technologies, announces its first full-scale shipment of antigen produced using its new...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Feb 13, 2020 | News Releases
Positive Cash Flow from Operations Achieved MISSISSAUGA, February 13, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), a life sciences innovator making critical ingredients that enable the production of clinical diagnostics and creating medical devices...
by Microbix | Feb 3, 2020 | Events
The AGM will still be held on March 31, 2020 at 1:00 pm, however due to the closure of our planned venue and alternative venues, we will be proceeding with access being provided online or via teleconference only. Due to the COVID-19 pandemic, Microbix also wants to...
by Microbix | Jan 31, 2020 | News Releases
TORONTO, October 19, 2017 – Microbix Biosystems Inc. (TSX: MBX) (“Microbix” or the “Company”), a developer and marketer of biological products and technologies, announces that it has completed a brokered private placement financing totaling $2.33 million dollars,...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Dec 20, 2019 | News Releases
Full-Year Sales of $13.4 Million, Progress Toward Sustained Profitability MISSISSAUGA, December 20, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), a life sciences innovator making critical ingredients that enable the production of clinical diagnostics...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Dec 4, 2019 | Events
CLICK TO...
by Microbix | Dec 2, 2019 | Events
IVD Controls Data at EUROGIN, December 4-7, 2019 MISSISSAUGA, December 2, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), a life sciences innovator making critical ingredients that enable the production of clinical diagnostics and creating medical devices...
by Microbix | Nov 18, 2019 | News Releases
2019 “OBAA” for Excellence in Export Strategy MISSISSAUGA, November 18, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), a life sciences innovator making critical ingredients that enable the production of clinical diagnostics and creating medical devices...
by Microbix | Oct 8, 2019 | Events
Two Abstracts on IVD Controls at EMMD, October 9-11, 2019 Download PDF Download MS Word
by Microbix | Oct 1, 2019 | Events
Meetings with Growth-Oriented Investors, October 3, 2019 MISSISSAUGA, October 1, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of clinical diagnostic materials and quality control medical devices, announces that it will be presenting to...
by Microbix | Sep 25, 2019 | Events
Meetings with Growth-Oriented Investors, September 27-29, 2019 MISSISSAUGA, September 25, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of clinical diagnostic materials and medical devices for test quality control, announces that it will be...
by Microbix | Sep 19, 2019 | News Releases
Four IVD Controls to Support Performance of Human Papilloma Virus (HPV) Tests MISSISSAUGA, September 19, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, is pleased to announce U.S. availability of four...
by Microbix | Sep 5, 2019 | News Releases
Four IVD Controls to Support Performance of Human Papilloma Virus (HPV) Tests MISSISSAUGA, September 5, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, is pleased to announce European registration for...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Aug 12, 2019 | News Releases
Q3 Sales of $3.1 Million and $9.8 Million Year-To-Date TORONTO, August 12, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, reports results for its third quarter and nine months of fiscal 2019, the...
by Microbix | Aug 1, 2019 | News Releases
Largest Customer Confirms Conversion Timetable & Provides Full-Scale Order TORONTO, August 1, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, provides an update on the conversion of its...
by Microbix | Jul 30, 2019 | News Releases
Contribution of $2.75 Million to Scale-up, Meet Demand, and Create Highly-Skilled Jobs MISSISSAUGA, July 30, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, is pleased to announce the execution of a...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Jul 9, 2019 | Events
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | May 13, 2019 | News Releases
Record Sales of $4.25 Million, Sales Growth of 42%, and Material Net Earnings TORONTO, May 13, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, reports results for its second quarter and first half of...
by Microbix | May 4, 2019 | Events
Microbix will be exhibiting at the 35th annual ASM/CVS (Clinical Virology Symposium) in Savannah on May 04-08th. We are showcasing our recently launched line of QAPs (Quality Assessment Products) used in a range of infectious disease diagnostics control applications....
by Microbix | Apr 29, 2019 | Events
Healthcare Investment Conference in Toronto on May 2 & 3, 2018 TORONTO, April 30, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces that it has been invited to present to investors about...
by Microbix | Apr 18, 2019 | News Releases
Ken Hughes Appointed COO, Kathryn Froh Retiring TORONTO, April 18, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces that it has appointed Dr. Kenneth (Ken) Hughes as its Chief Operating...
by Microbix | Mar 28, 2019 | News Releases
TORONTO, March 28, 2019 – Microbix Biosystems Inc. (TSX: MBX, “Microbix” or the “Company”), an innovator of biological products and technol…
by Microbix | Mar 18, 2019 | Events
Microbix will be exhibiting at the 29th annual ECCMID (European Congress of Clinical Microbiology and Infectious Disease) in Amsterdam on April 13-16th. We are showcasing our recently launched line of QAPs™ (Quality Assessment Products) used in a range of infectious...
by Microbix | Mar 5, 2019 | Events
Shareholders and other interested parties, please note Microbix will hold its annual general meeting (AGM) at the Toronto University Club located at 380 University Avenue, Toronto, M5G 1R6 on Wednesday March 27th at 1 PM. Click here for INFO CIRCULAR
by Microbix | Feb 25, 2019 | News Releases
Reading Time: 3 minutes Using Shareholder-Approved Plan to Incentivize and Retain TORONTO, February 25, 2020 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), a life sciences innovator making critical ingredients that enable the production of clinical...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Feb 12, 2019 | News Releases
Lower Sales, Improving Gross Margin, Positive Cash Flow from Operations TORONTO, February 12, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, reports results for its first quarter of fiscal 2019, the...
by Microbix | Feb 5, 2019 | News Releases
New Customers, More Products, and Expanding Capacity TORONTO, February 5, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, provides an update on the expansion of its quality assessment product lines,...
by Microbix | Dec 24, 2018 | News Releases
ISO 13485:2016 Medical Devices Certification Attained TORONTO, December 24, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces that it has attained the ISO 13485:2016 Medical Devices...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Dec 21, 2018 | News Releases
Full-Year Sales Growth of 23%, Progress Toward Sustained Profitability TORONTO, December 21, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, reports results for its fourth quarter of fiscal 2018, the...
by Microbix | Nov 19, 2018 | News Releases
Recognized with 2018 Business Innovation Award by MBOT TORONTO, November 19, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces that it has been recognized with the 2018 Business Innovation...
by Microbix | Nov 6, 2018 | Events
Showcasing Antigen and Quality Assessment Products TORONTO, November 6, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces that it will be exhibiting its antigens and quality assessment products...
by Microbix | Sep 27, 2018 | Events
TORONTO, September 27, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces that it will be presenting to investors about Microbix at the Muskoka Capital Conference, organized by Capital Event...
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Aug 14, 2018 | News Releases
Microbix Reports Record Third Quarter Sales Year-Over-Year Sales Growth of 24% in First Nine Months TORONTO, August 14, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, reports financial results for...
by Microbix | Jul 26, 2018 | Events
Showcasing New Quality Assessment Products TORONTO, July 26, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces that it will be exhibiting its Quality Assessment Products (QAPs™) at the 70th...
by Microbix | Jul 12, 2018 | Events
Conference Introduces Growth Companies to Investors, July 12-14, 2018 TORONTO, July 12, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces that it will be presenting to investors about Microbix...
by Microbix | May 14, 2018 | News Releases
Microbix Reports Record Second Quarter Sales
by Microbix | May 8, 2018 | News Releases
Production Capacity Increased to Support Growth Objectives TORONTO, May 8, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces the completion of multiple upgrades to its production facility, to...
by Microbix | Apr 30, 2018 | Events
Healthcare Investor Conference in Toronto on April 30 & May 1 TORONTO, April 29, 2019 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces that its CEO, Cameron Groome, has been invited to present...
by Microbix | Apr 26, 2018 | News Releases
Torreya Partners to Assist with Kinlytic® urokinase TORONTO, April 26, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces the engagement of Torreya Partners LLC of New York to assist it in...
by Microbix | Apr 3, 2018 | News Releases
TORONTO, April 2, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces th…
by Microbix | Apr 2, 2018 | Events
Join us at ECCMID in Madrid in April. It will be our first time exhibiting there and our QAPs™ lines will be our highlighted. For more information, visit http://www.eccmid.org/
![Microbix Reports Results for Q1 Fiscal 2023 – Sales of $2.5 million, Net Loss of $1.3 million]()
by Microbix | Feb 14, 2018 | News Releases
TORONTO, February 14, 2018 ‐ Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, reports financial results for its first quarter of fiscal 2018, the three‐ month period ending December 31, 2017 (“Q1”), with record...
by Microbix | Jan 29, 2018 | Events
Invited onto Panel about Managing Canadian Healthcare Companies for Growth TORONTO, January 29, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces its participation as a panelist and exhibitor...
by Microbix | Jan 29, 2018 | Events
JANUARY 29, 2018 Celebrating the 5th anniversary of Canada’s largest technology investment conference. We are excited to bring over 3500 engaged investors together with 100+ leading technology companies. Visit us at the Metro Toronto Convention Center on January 31...
by Microbix | Jan 18, 2018 | News Releases
48 New Products Being Added, Under PTDx™ and PROCEEDx™ Brands TORONTO, January 18, 2018 – Microbix Biosystems Inc. (TSX: MBX, Microbix®), an innovator of biological products and technologies, announces the expansion of its quality assessment product...

by Microbix | Dec 21, 2017 | News Releases
TORONTO, December 21, 2017 – Microbix Biosystems Inc. (TSX: MBX), an innovator of biological products and technologies, reports financial results for its fiscal year and fourth quarter ended 30 September, 2017 (“2017” and “Q4”). Management Discussion In 2017, the...
by Microbix | Nov 27, 2017 | News Releases
TORONTO, November 27, 2017 – Microbix Biosystems Inc. (TSX: MBX), a developer and marketer of biological products and technologies, confirms its production ramp-up, with a 500% increase in bioreactor capacity and other processing upgrades. Microbix’ revenues are...
by Microbix | Oct 27, 2017 | News Releases
TORONTO, October 27, 2017 – Microbix Biosystems Inc. (TSX: MBX) (“Microbix” or the “Company”), a developer and marketer of biological products and technologies, announces that it has completed a second and final tranche of the brokered private placement financing...
by Microbix | Oct 19, 2017 | News Releases
TORONTO, October 19, 2017 – Microbix Biosystems Inc. (TSX: MBX) (“Microbix” or the “Company”), a developer and marketer of biological products and technologies, announces that it has completed a brokered private placement financing totaling $2.33 million dollars,...
by Microbix | Oct 11, 2017 | News Releases
OCTOBER 11, 2017 TORONTO, October 11, 2017 – Microbix Biosystems Inc. (TSX: MBX), an innovator of biological products and technologies, is pleased to announce the withdrawal of legal claims alleging patent infringement that were filed in Canadian Federal Court against...
by Microbix | Sep 13, 2017 | News Releases
Customer Validation Of New Manufacturing Process Completed TORONTO: September 13, 2017 – Microbix Biosystems Inc. (TSX: MBX), a developer and marketer of biological products and technologies, announces its first full-scale shipment of antigen produced using its new...
by Microbix | Aug 24, 2017 | News Releases
TORONTO August 24 2017 – Microbix Biosystems Inc. (TSX: MBX), a developer and marketer of biological products and technologies, announces it has applied to the TSX to amend the terms of 1,500,000 common share purchase warrants (the “Warrants”) issued in connection...
by Microbix | Aug 14, 2017 | News Releases
TORONTO, August 14, 2017 – Microbix Biosystems Inc. (TSX: MBX), an innovator of biological products and technologies, today reports financial results for its fiscal third quarter and nine months ended June 30, 2017 (“Q3” and “9 Mo.”). Third Quarter Financial Results...









