Record Revenues of $8.4 million and Record Net Income of $2.5 million
MISSISSAUGA, CANADA, February 14, 2024 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, reports results for its first quarter of fiscal 2024 ended December 31, 2023 (“Q1”), with record quarterly revenues and a record quarterly profit, reflective of ongoing progress to increase sales from its diagnostic-test related ingredients and devices, and material licensing revenues from its fully-funded program to revalidate and relaunch its approved drug, Kinlytic® urokinase (“Kinlytic”).
Management Discussion
Results for Q1 demonstrate strong growth in sales of each of Microbix’s test ingredients (“Antigens”) and its test quality assessment products (“QAPs™”), which were collectively up by 80% year-over-year. In addition, Microbix recognized and received material licensing payments relating to Kinlytic. Collectively, the resulting revenues of over C$ 8.4 million led to strong net earnings and set the stage for a record full-year fiscal 2024. Microbix believes sales growth will continue for Antigens and QAPs, alongside satisfaction with the progress of Kinlytic toward FDA re-approval and re-launch into the United States market.
Quarter Ending December 31, 2023 (“Q1”)
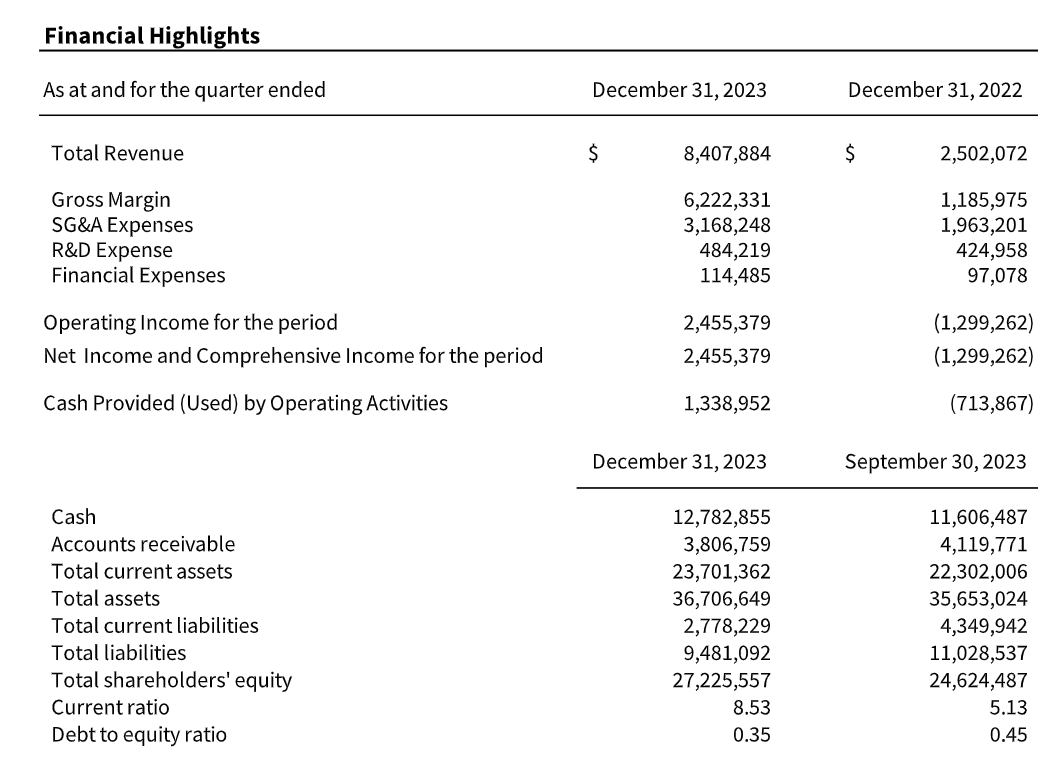
Q1 revenue was $8,407,884, a 236% increase from Q1 2023 revenues of $2,502,072. Antigen sales grew by 95% to $1,953,677 (Q1 F2023 – $1,003,608), while QAPs grew by 69% to $2,248,236 (Q1 F2023 – $1,333,503). Revenue from royalties decreased to $119,311 (Q1 F2023 – $164,762). Q1 revenues were also greatly influenced by the recognition of $4,086,000 in Kinlytic licensing milestone payments (Q1 F2023 – nil).
Q1 gross margin was 74%, significantly up from Q1 2023 gross margins of 47%. Gross margins were primarily impacted by Kinlytic licensing revenues, to which no COGS were attached. Without the impact of the Kinlytic licensing revenues, our gross margins of 49% were up from 47% last year.
Operating and finance expenses in Q1 increased by 52% relative to Q1 2023 principally due to consulting fees related to our Kinlytic licensing agreement that were absorbed into G&A. In addition, Q1 costs reflect the ongoing costs of our new IT systems which began in the latter half of fiscal 2023, and amortization relating to the reversal of the impairment of the Kinlytic intangible asset which began at the end of fiscal 2023.
Increased sales and higher gross margins were partially offset by increased operating expenses (due to increased investment into business growth and infrastructure). The above results led to a Q1 operating income and net income of $2,455,379 versus a Q1 2023 operating loss and net loss of ($1,299,262). Cash provided by operating activities was $1,338,952, compared to cash used in operating activities of ($713,867) in Q1 2023.
At the end of Q1, Microbix’s current ratio (current assets divided by current liabilities) was 8.53 and its debt to equity ratio (total debt over shareholders’ equity) was 0.35, both measures having improved from the prior year first quarter (Q1 2023) and the immediately preceding fourth quarter (Q4 2023).
Corporate Outlook
Microbix will continue to drive sales growth across all of its business lines, and work to keep improving percentage gross margins and driving bottom-line results. Management currently expects Microbix to generate meaningful year-over-year growth in revenues and net earnings across full-year fiscal 2024.
Adelaide Capital will host a live webinar with management, on Wednesday, February 14 at 10am ET. Please register here: https://us02web.zoom.us/webinar/register/WN_N3yHVFwcQNq0bA3MeJHPSg .
It will also be live-streamed to YouTube at: https://www.youtube.com/channel/UC7Jpt_DWjF1qSCzfKlpLMWw.
A replay of the webinar will also be made available on Adelaide Capital’s YouTube channel
About Microbix Biosystems Inc.
Microbix Biosystems Inc. creates proprietary biological products for human health, with over 100 skilled employees and annualized sales targeting C$ 2.0 million per month. It makes a wide range of critical ingredients and devices for the global diagnostics industry, notably antigens for immunoassays and its laboratory quality assessment products (QAPs™) that support clinical lab proficiency testing, enable assay development and validation, or help ensure the quality of clinical diagnostic workflows. Its antigens drive the antibody tests of approximately 100 diagnostics makers, while QAPs are sold to clinical lab accreditation organizations, diagnostics companies, and clinical labs. Microbix QAPs are now available in over 30 countries, supported by a network of international distributors. Microbix is ISO 9001 & 13485 accredited, U.S. FDA registered, Australian TGA registered, Health Canada establishment licensed, and provides CE marked products.
Microbix also applies its biological expertise and infrastructure to develop other proprietary products and technologies, most notably Kinlytic® urokinase, a biologic thrombolytic drug used to treat blood clots, and viral transport medium (DxTM™), to stabilize patient samples for lab-based molecular diagnostic testing. Microbix is traded on the TSX and OTCQX, and headquartered in Mississauga, Ontario, Canada.
Forward-Looking Information
This news release includes “forward-looking information,” as such term is defined in applicable securities laws. Forward-looking information includes, without limitation, discussion of financial results or the outlook for the business, risks associated with its financial results and stability, its current or future products, development projects such as those referenced herein, sales to foreign jurisdictions, engineering and construction, production (including control over costs, quality, quantity and timeliness of delivery), foreign currency and exchange rates, maintaining adequate working capital and raising further capital on acceptable terms or at all, and other similar statements concerning anticipated future events, conditions or results that are not historical facts. These statements reflect management’s current estimates, beliefs, intentions, and expectations; they are not guarantees of future performance. The Company cautions that all forward looking information is inherently uncertain, and that actual performance may be affected by many material factors, some of which are beyond the Company’s control. Accordingly, actual future events, conditions and results may differ materially from the estimates, beliefs, intentions, and expectations expressed or implied in the forward-looking information. All statements are made as of the date of this news release and represent the Company’s judgement as of the date of this new release, and the Company is under no obligation to update or alter any forward-looking information.
Please visit www.microbix.com or www.sedarplus.ca for recent Microbix news and filings.
For further information, please contact Microbix at:
| Cameron Groome, CEO (905) 361-8910 |
Jim Currie, CFO (905) 361-8910 |
Deborah Honig, Investor Relations Adelaide Capital Markets (647) 203-8793 ir@microbix.com |
Copyright © 2024 Microbix Biosystems Inc.
Microbix®, DxTM™, Kinlytic®, & QAPs™ are trademarks of Microbix Biosystems Inc.
[/et_pb_text][/et_pb_column][/et_pb_row][/et_pb_section]

