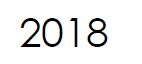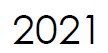|
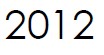
|
Microbix appoints Vaughn Embro-Pantalony as the new CEO & President.
Mr. Embro-Pantalony previously held senior executive positions in large biopharmaceutical and agriculture companies, including VP, Finance & CFO at Novopharm Limited, VP, Information Technology & CIO at Bayer Inc., VP, Finance & Administration at Bayer Healthcare, and General Manager of Terra International. |
|
Microbix signs its first Molecular Diagnostics Agreement with Microbiologics Inc. The partnership combines the companies’ expertise in biomaterial development, preservation, and distribution to create highly stable viral controls for molecular testing.
|
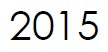
|
 |
 |
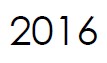
|
Microbix confirms its production ramp-up, with a 500% increase in bioreactor capacity and other processing upgrades to improve the efficiency, capacity and reliability of its rubella products.
|
|
Microbix appoints Meridian Life Science, Inc. (a wholly-owned company of Meridian Bioscience, Inc.) as exclusive distributor in Asia-Pacific.
Meridian receives exclusive distribution rights to Microbix antigen products for China, Hong Kong, Taiwan and Macau.
|
|
 |
 |
|
Cameron Groome is appointed the new CEO of Microbix.
Before joining Microbix, he was the CEO of a company listed on the TSX Venture Exchange and EVP of a TSX-listed company, both in life sciences. Before his operational roles, he headed the healthcare activities of two national investment dealers. He has more than 25 years of experience as a leader, executive, equity analyst, investment banker, and corporate advisor. He has also been a member of the Life Sciences Advisory Group of Global Affairs Canada, and a director of public and private companies. |
|
Microbix launches its new Quality Assessment products (QAPs™), manufactured from native pathogens or synthetic whole genome of pathogens - to represent a very close facsimile of patient samples.
Microbix adds the PROCEEDx™ line to its quality assessment offerings, for use in research, assay development, validation / verification of instruments, troubleshooting, and operator training.
|
|
 |
|
Microbix wins the 2018 Business Innovation Award in recognition of innovations relating to manufacturing, development, and international commercialization of biological products for the diagnostics industry.
|
|
 |
 |
|
Microbix expands its new Quality Assessment products (QAPs™). All QAPs™ are manufactured from native pathogens or synthetic whole genome of pathogens, representing very close facsimiles to patient samples.
Microbix adds the REDx™ controls line to its portfolio, intended to evaluate laboratory testing performance, procedures, and workflow in nucleic acid and/or antigen-based assays that detect infectious disease pathogens in patient samples. |
|
 |
REDx™ Controls are designed to control the diagnostic process by detecting deviations from desired assay performance. All REDx™ controls are manufactured under ISO 13485: 2016 standard, FDA 21 CFR Part 820 Regulations and CE-marked under EU regulations. They are available for purchase in Canada, USA, Europe, UK and Australia.
|
In order to effectively support its QAPs™ programs, Microbix announces a long term lease of an additional office, production, and warehousing space at 235 Watline Avenue in Mississauga.
The newly-leased space further provides 10,300 square feet of space adjacent to its fully-owned 14,000 square foot production site at 265 Watline Avenue. |
|
 |
2020s
Microbix and Copan Italia S.p.A. (Copan®), the global leader in specimen collection technologies, announce the execution of a strategic agreement with Microbix’s quality assessment products (QAPs™) and Copan’s flocked devices (FLOQ®).
This combines Copan’s well-known FLOQ® brand with Microbix’s emerging PROCEEDx™ (RUO) and REDx™ (IVD) brands – to create PROCEEDx™ FLOQ® and REDx™ FLOQ® as worldwide branding for active co-marketing of leading-edge FLOQSwab-formatted QAPs™ by Microbix and Copan. |
 |
 |
|

|
|
William J. Gastle, Microbix’s founder, and former CEO and Chairman of the Board, retires. |
| Microbix’s receives a grant of $1.45 million from the Ontario Together Fund to cover 50% of the cost to scale-up production of two emerging Microbix product lines – Viral Transport Medium (now branded as DxTM™), and Quality Assessment Products (QAPs™) to assist in managing the pandemic across Canada, Australia, the EU, Scandinavia, the UK, and the US. |
|
 |
Microbix receives a $4.25 million order for its viral transport medium (generically known as “VTM” and branded as “DxTM™” by Microbix).
DxTM is a test sample-collection device that is essential for PCR-testing for the virus causing COVID-19 disease. |
|

|
 |
 |
Microbix announces collaboration with SpeeDx Pty. Ltd (SpeeDx), under which Microbix will be exclusive developer of Quality Assessment Products (QAPs™) to facilitate the registration and commercialization of SpeeDx diagnostic assays.
|
| Microbix secures a third manufacturing site in Mississauga, Ontario, a long-term lease agreement for a property adjacent to its two current sites. This site is reconfigured and equipped to support additional business activities, including implementation of further scale-up, full automation of production processes for its DxTM™ branded viral transport medium (generically known as “VTM”), and commissioning of additional space for other fully-automated production processes, including vial-formatted or FLOQSwab®-formatted quality assessment products (QAPs™). |
|
 |
|