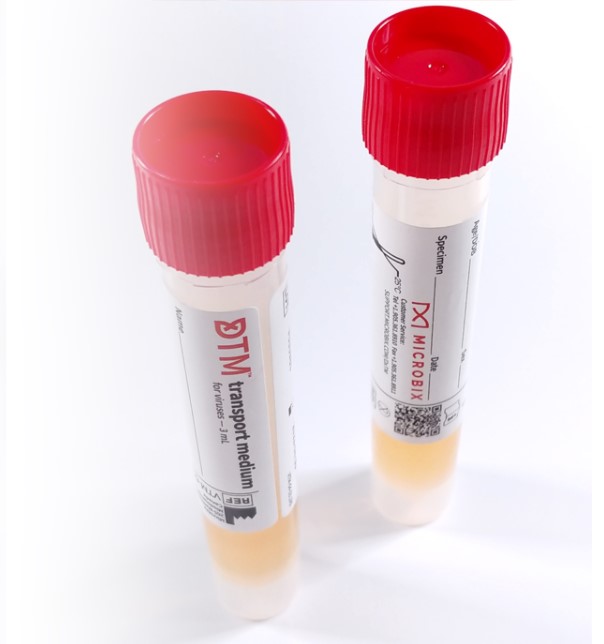
DxTM™ Viral Transport Medium
Microbix’s DxTM™ Viral Transport Medium is a Canadian-made, proprietary IVD Medical Device that is designed for use in the collection and transport of specimens containing viruses, including SARS-CoV-2. The DxTM™ formulation is designed to stabilize available viral particles within a given sample. It is intended for the collection and transport of clinical specimens containing viruses from the collection site to the testing clinical laboratory. DxTM™ is suitable for processing using standard procedures for viral nucleic acid testing.
DxTM™ is used primarily for transporting patient sample swabs or for transporting materials released into the medium from such swabs. Manufactured under our ISO 13485 Quality Management System, DxTM™ packaging tubes contain 1 or 3 ml of the medium, with the tubes able to accommodate 80 mm and 100 mm breakpoint swabs for transportation. DxTM™ facilitates the collection and transport of samples in areas where refrigeration is not readily available, with validated ambient temperature storage and shipping (2-25°C). The advanced formulation stabilizes viral components and suppresses microbial contamination.
DxTM™ Viral Transport Medium is validated for viral nucleic acid testing. It is designed to be non-inactivating so it could also potentially be used for isolating and growing viral pathogens from patient specimens. This was performed in comparison to the Gold-Standard predicate device, where DxTM™ was shown to be equivalent to that Gold-Standard in terms of handling and extraction, thereby assuring the very highest standard of performance.