Strong Sales of $5.5 million alongside Drug Partnering Agreement
MISSISSAUGA, August 10, 2023 – Microbix Biosystems Inc. (TSX: MBX, OTCQX: MBXBF, Microbix®), a life sciences innovator, manufacturer, and exporter, reports results for its third quarter and YTD fiscal 2023 ended June 30, 2023 (“Q3” and “YTD”), a period in which strong revenues were realized along with a fully-funded partnering of its drug asset Kinlytic® urokinase (“Kinlytic”).
Management Discussion
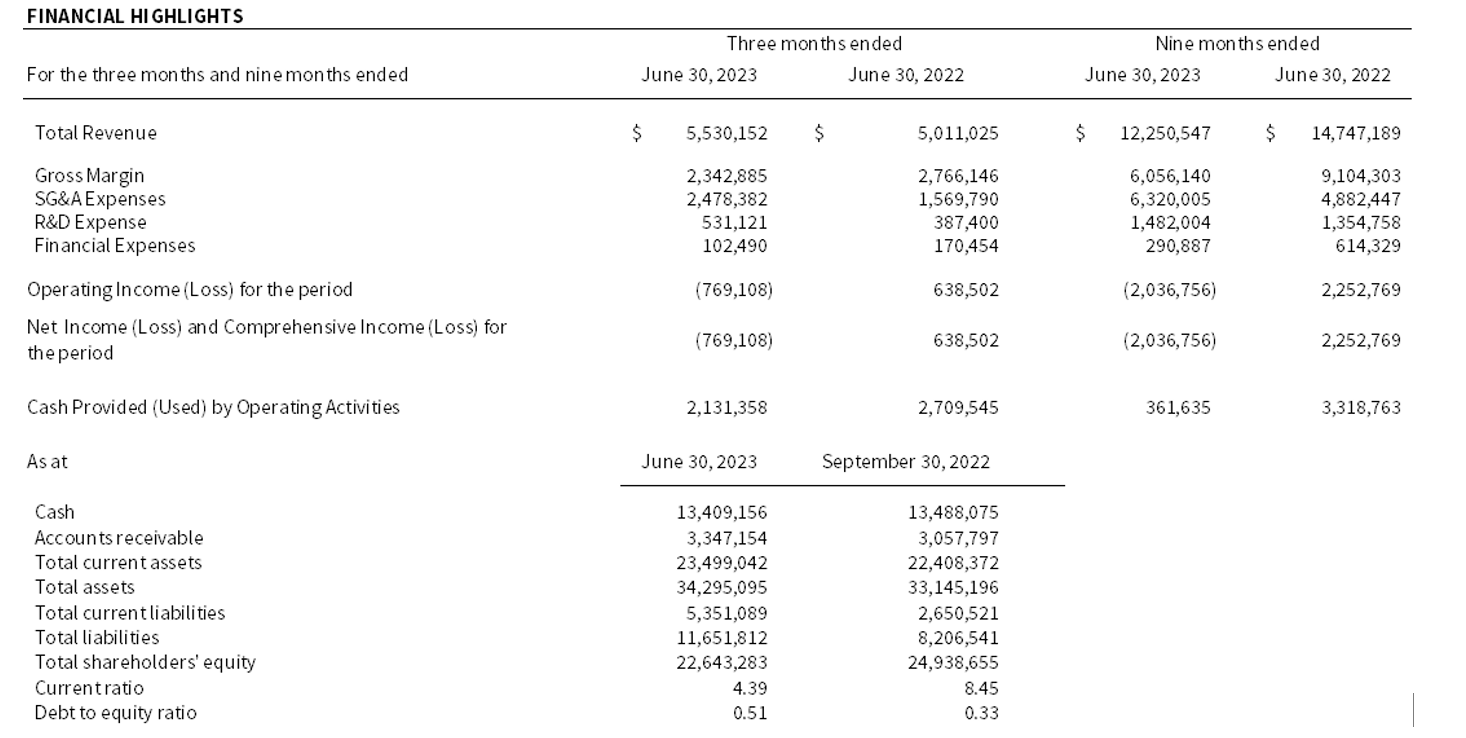
Q3 posted revenues of $5.5 million, with continued strength in sales of test ingredients (“Antigens”) and test-controls (“QAPs™”) totaling $4.0 million, but none of sample collection media (“DxTM™”). Revenues in Q3 benefited from the recognition of over $1.3 millon of Kinlytic license fees, however this was more than offset by a write-down of aging DxTM inventory and other expense increases totaling $2.2 million. Such expenses resulted in an off-trend net loss of $0.8 million for Q3. Microbix remains well-capitalized and continues to build its customer relationships and systems with the goal of reaching annual sales of $100 million within several years.
For the three months ending June 30, 2023 (“Q3”)
Q3 revenue was $5,530,152, an increase of 10% from Q3 2022 revenues of $5,011,025. Antigen sales of $2,608,521 grew 14% versus Q3 2022 ($2,283,621), while QAPs sales were $1,456,905 (2022 – $1,305,896). In turn, revenue from DxTM was zero in Q3 (2022 – $1,326,410) and royalties were $116,226 (2022 – $95,099). In Q3, there were also Kinlytic-related revenues of $1,348,500 recognized from a licensing and funding agreement (“Agreement”) that was announced on May 16, 2023. Agreement-related licensing revenue largely offset the lack of Ontario-driven deliveries of DxTM in Q3 compared to the prior year.
Q3 gross margin was 42%, down from 55% in Q3 2022. This decline was due to a write-down of aging DxTM inventory of close to $1.0 million and a product-mix related increase to cost-of-goods-sold (“COGS”) of $0.6 million relative to Q3 2022. Beyond COGS, operating expenses (“OpEx”) in Q3 increased by 46% relative to Q3 2022. OpEx increases were in large part due to increased investment in IT infrastructure to support our continuing growth objectives – software start-up costs relating to our “ERP” and “eQMS” implementations. Such IT systems start-up costs were heaviest in Q3, as Microbix drove toward a Q4 2023 “go-live” for its new ERP system. Along with prior-year foreign exchange gains that were replaced by losses, such costs totaled $0.3 million in Q3, to which were added $0.4 million of consulting fees and expenses relating to the Kinlytic Agreement. Finance expenses were lower than the prior year due to repayment of debentures and long-term debt during fiscal 2022 and higher returns from short-term investment of cash balances.
Overall, Q3 sales led to an operating and net loss of $769,108 versus a Q3 2022 operating and net income of $638,502. Cash provided by operating activities remained strong at $2,131,358 in Q3 compared to cash provided by operating activities of $2,709,545 in Q3 2022, with the relative reduction coming from a greater deployment of cash into working capital account balances during the quarter.
Nine Months Ending June 30, 2023 (“YTD”)
YTD revenue was $12,250,547, with a 17% decrease from YTD 2022 due to a lack of DxTM sales. Included were Antigen sales of $6,615,040, up 17% from last year ($5,658,007). QAPs revenues of $3,892,090 were largely flat from YTD 2022 ($3,773,429), due in large-part to delays in the test finalization and launch timelines of customers intending to include Microbix QAPs in their kits of test consumables. YTD Kinlytic revenues were $1,350,517 compared to zero in YTD 2022, which is due to the announced Agreement. In turn, YTD revenue from DxTM was zero (2022 – $ 5,004,359) due to agents of the Province of Ontario unexpectedly returning to imported product for all domestic needs. Finally, YTD royalties were $392,898 (2022 – $ 311,394). In summary, the lower YTD sales result was driven by the lack of any deliveries of DxTM for the Province of Ontario.
YTD gross margin was 49%, down from 62% in YTD 2022, due to the lack of DxTM sales, the material writedown of DxTM inventory in Q3 and the effects of a greater proportion of antigen sales that have lower margins than QAPs or DxTM. In addition, we continue to see double-digit materials price increases across our supply chain, which take time to pass-through in product pricing to Microbix customers.
Operating expenses in YTD increased by 18% relative to YTD 2022, due to increased investment in IT infrastructure, unfavourable foreign exchange impact vs. 2022 and the recognition of Kinlytic Agreement costs. This was partly offset by lower finance expenses due to repayment of debentures and long-term debt during fiscal 2022 and short-term investment of cash balances. Overall, weaker YTD sales led to an operating loss and net loss of $2,036,756 versus a YTD 2022 operating income and net income of $2,252,769. Cash provided by operating activities YTD was $361,635, compared to cash provided by operating activities of $3,318,763 in YTD 2022, with much of the change coming from the change in operating income and the repurchase of shares through our Normal-Course Issuer Bid, which used $0.9M YTD.
At the end of Q3, Microbix’s current ratio (current assets divided by current liabilities) was 4.39 and its debt-to-equity ratio (total debt over shareholders’ equity) was 0.51.
Corporate Outlook
Microbix continues working to grow revenues across its business lines, and to improve gross margins to drive bottom-line results. Management remains committed to a profitable growth model, a goal that should be aided by the Agreement to advance Kinlytic to a successful re-launch into the U.S. market.
Microbix continues working to grow revenues across its business lines, and to improve gross margins to drive bottom-line results. Management remains committed to a profitable growth model, a goal that should be aided by the Agreement to advance Kinlytic to a successful re-launch into the U.S. market
Adelaide Capital will host a live webinar with management on Monday, August 14 at 11am ET. Please register here: https://us02web.zoom.us/webinar/register/WN_xUe4T25HQgmOOFDp6B8Icg. It will also be live streamed to YouTube at: https://www.youtube.com/channel/UC7Jpt_DWjF1qSCzfKlpLMWw.
A replay of the webinar will also be made available on Adelaide Capital’s YouTube channel.
About Microbix Biosystems Inc.
Microbix Biosystems Inc. creates proprietary biological products for human health, with over 100 skilled employees and annualized sales targeting C$ 2.0 million per month. It makes a wide range of critical ingredients and devices for the global diagnostics industry, notably antigens for immunoassays and its laboratory quality assessment products (QAPs™) that support clinical lab proficiency testing, enable assay development and validation, or help ensure the quality of clinical diagnostic workflows. Its antigens drive the antibody tests of approximately 100 diagnostics makers, while QAPs are sold to clinical lab accreditation organizations, diagnostics companies, and clinical labs. Microbix QAPs are now available in over 30 countries, supported by a network of 10 international distributors. Microbix is ISO 9001 & 13485 accredited, U.S. FDA registered, Australian TGA registered, Health Canada establishment licensed, and provides CE marked products.
Microbix also applies its biologics expertise and infrastructure to develop other proprietary products and technologies, most notably viral transport medium (DxTM™) to stabilize patient samples for lab-based molecular diagnostic testing and Kinlytic® urokinase, a thrombolytic drug used to treat blood clots. Microbix is traded on the TSX and OTCQX, and headquartered in Mississauga, Ontario, Canada.
Forward-Looking Information
This news release includes “forward-looking information,” as such term is defined in applicable securities laws. Forward-looking information includes, without limitation, discussion of financial results or the outlook for the business, risks associated with its financial results and stability, its current or future products, development projects such as those referenced herein, sales to foreign jurisdictions, engineering and construction, production (including control over costs, quality, quantity and timeliness of delivery), foreign currency and exchange rates, maintaining adequate working capital and raising further capital on acceptable terms or at all, and other similar statements concerning anticipated future events, conditions or results that are not historical facts. These statements reflect management’s current estimates, beliefs, intentions and expectations; they are not guarantees of future performance. The Company cautions that all forward looking information is inherently uncertain and that actual performance may be affected by a number of material factors, many of which are beyond the Company’s control. Accordingly, actual future events, conditions and results may differ materially from the estimates, beliefs, intentions and expectations expressed or implied in the forward-looking information. All statements are made as of the date of this news release and represent the Company’s judgement as of the date of this new release, and the Company is under no obligation to update or alter any forward-looking information.
Please visit www.microbix.com or www.sedarplus.ca for recent Microbix news and filings.
For further information, please contact Microbix at:
| Cameron Groome, CEO (905) 361-8910 |
Jim Currie, CFO (905) 361-8910 |
Deborah Honig, Investor Relations Adelaide Capital Markets(647) 203-8793 ir@microbix.com |
Copyright © 2023 Microbix Biosystems Inc. Microbix®, DxTM™, Kinlytic®, and QAPs™ are trademarks of Microbix Biosystems Inc



